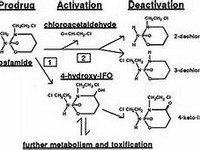
Aluminium hydroxide

Aluminium hydroxide
CLINICAL USE
Phosphate binding agent
Antacid
DOSE IN NORMAL RENAL FUNCTION
Phosphate binder: 4–20 capsules daily in
divided doses
Antacid: 1 capsule 4 times daily and at
bedtime
PHARMACOKINETICS
Molecular weight :
78
%Protein binding :
70–90
%Excreted unchanged in urine :
No data
Volume of distribution (L/kg) :
No data
half-life – normal/ESRD (hrs) :
No data
DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
20 to 50 : Dose as in normal renal function
10 to 20 : Dose as in normal renal function
<10 :
Dose as in normal renal function
DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
CAPD :
Unknown dialysability. Dose as in
normal renal function
HD :
Unknown dialysability. Dose as in
normal renal function
HDF/high flux :
Unknown dialysability. Dose as in
normal renal function
CAV/VVHD :
Unknown dialysability. Dose as in
normal renal function
IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugs
None known
ADMINISTRATION
Reconstition
–
Route
Oral
Rate of Administration
–
Comments
–
OTHER INFORMATION
K/DOQI guidelines caution that CKD 5
patients on chronic therapy may develop
aluminium toxicity; therefore best avoided
in all but short-term therapy (calcium
carbonate, calcium acetate, lanthanum or
sevelamer are used in chronic therapy)
Take/administer with or immediately
before meals
In patients undergoing chronic therapy
with aluminium hydroxide, serum
aluminium levels should be monitored
See how to identify renal failure stages according to GFR calculation
See how to diagnose irreversible renal disease
Home